Three Big Questions for Stage 3 & Patient Engagement
Chilmark Research
JANUARY 17, 2014
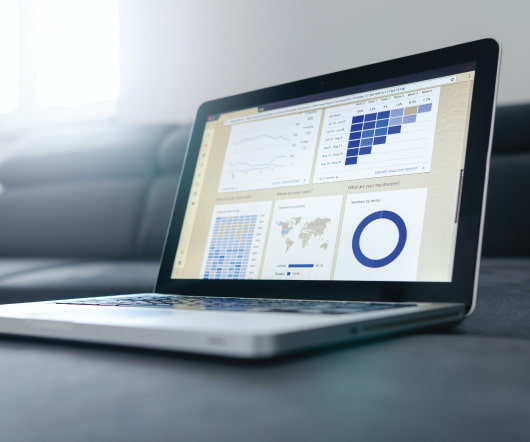
For many, the delay of Stage 3 of the Meaningful Use program evoked a collective sigh of relief, providing a much-needed extra year to focus on the challenging requirements for patient engagement and interoperability. Each of these use cases brings with it a unique set of programmatic and technical components. In a word, no.










Let's personalize your content