Finding the future of interoperability with Redox: Part 3 – How Carequality can solve disconnected data for digital health
Redox
MAY 6, 2024
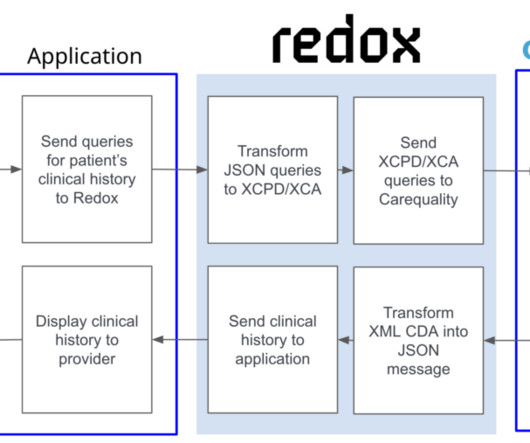
healthcare providers via a network approach—with one business relationship and one integration go-live—as opposed to building many BAA-driven relationships and integrations with individual healthcare organizations and their EHRs. new test results) or you need to be able to write data back to their charts in EHRs.















Let's personalize your content