A whole new way to develop medicines has grown up around biotherapeutics, of which the best-known are a subset called biosimilars. Over the past ten years, a class of biotherapeutics called cell and gene therapies can even provide cures for previously untreatable diseases such as inherited genetic diseases.
The development and manufacturing process for these important new drugs is very different from traditional medicines. This article focuses on the technology that ensures quality in the process, and is based on a conversation with Graziella Piras, PhD, Life Science Business Development Director at 908 Devices.
Traditional drugs are built from molecules synthesized chemically, combining the active ingredients with other substances that protect the molecules and ensure that they’re delivered to the right part of the body after being swallowed, injected, or otherwise applied.
Biotherapeutics, in contrast, are manufactured by living cells; sometimes the cells themselves are the drug. Biosimilars are just biotherapeutics that can replace other drugs, like generic medications.

The cells are grown in vitro in stainless steel, glass, or plastic vessels known as bioreactors. The bioreactors can be quite small during research and development (see Figure 1), and perhaps hold 2,000 to 20,000 liters in manufacturing. The vessels remain closed in order to prevent contamination, but contain ports to facilitate the sampling and feeding of the cells.

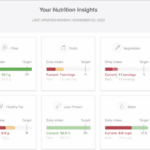
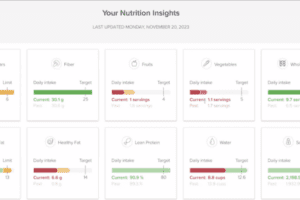
During research, the scientists determine the optimal feeding conditions for the cells to grow and thrive using a culture medium that provides a source of energy and other nutrients. Elements of the medium include glucose, amino acids, vitamins, and other metabolites. Monitoring is crucial during both development and manufacturing in order to maintain ideal conditions (Figure 2). If the conditions depart from the norm, the cells could die or could produce output that fails to work as medicine.
Real-time feedback from the cell culture environment is critical, supplemented by automated responses that can increase or decrease a certain condition. For instance, more glucose can be added if the level drops in the bioreactor. The feedback control prevents the cells from being starved, ensuring that the cells stay happy and produce high amounts of the biotherapeutic drug with the right form.
908 Devices offers a broad spectrum of products for chemical and biochemical analysis. Typically, the devices used for biotherapeutic development monitor, control, and optimize cell cultures (Figure 3).

Some systems that monitor cells require samples to be extracted manually. A new product from 908 Devices provides aseptic sampling. On-line monitoring is faster and safer, lessening the risk of contamination, and there’s no need to reduce output by extracting material from the vessel.
The biotherapeutics monitoring equipment from 908 Devices illustrates the positive role technology—sensitive measuring devices, analytics, and automation—can play in medicine. Here they are producing more accurate and efficient tools for drug development.