Imagine a healthcare landscape where treatment is accessible, patients receive personalized care tailored to their unique needs, and innovative approaches pave the way for improved medical outcomes. With the recent amendments to federal regulations governing opioid treatment programs (OTPs), this vision becomes a step in the right direction toward making this a reality.
Background and Purpose of 42 CFR Part 8
Established under 42 CFR Part 8, these regulations provide a comprehensive framework for the treatment and management of opioid use disorder (OUD) using opioid agonist medications such as methadone, buprenorphine, and naltrexone.
Outlined in the regulations are the standards and procedures necessary for OTPs to operate effectively while ensuring the safety and well-being of patients. Key components of these regulations include:
- Accreditation and Certification: OTPs must obtain certification from the Substance Abuse and Mental Health Services Administration (SAMHSA) to dispense opioid agonist medications.
- Patient Evaluation and Treatment: The regulations specify criteria for patient evaluation, admission, and ongoing treatment.
- Dispensing of Medications: OTPs are authorized to dispense FDA-approved opioid agonist medications for the treatment of OUD, including methadone, buprenorphine, and naltrexone.
- Compliance and Oversight: Compliance with federal regulations is monitored through regular inspections and oversight activities overseen by SAMHSA and other relevant agencies.
- Flexibility and Telehealth: In response to evolving healthcare needs and public health emergencies such as the COVID-19 pandemic, the regulations allow for flexibility in treatment delivery
In this article, we’ll explore the recent key changes to 42 CFR Part 8 and their implications for healthcare professionals striving to combat OUD.
42 CFR Part 8 Final Rule Changes and What They Mean for Treatment
The changes made to 42 CFR Part 8 highlight a notable shift in opioid treatment strategies and align with evolving patient behavior and how patients prefer to receive care. Updating terminology and standards reflects SAMHSA’s concerted effort to align treatment practices with current evidence-based approaches and patient preferences, fostering a more patient-centric approach to care. Eliminating barriers such as the 1-year opioid addiction history requirement and promoting priority treatment for pregnant individuals further emphasizes a commitment to inclusivity and equitable access to care.
As practitioners are well aware, substance abuse disorders require a multi-faceted approach to care, meeting patients where they are in their recovery journey and straying from a one-size-fits-all approach. The incorporation of harm reduction principles and shared decision-making into treatment acknowledges the need for individualized care. These regulatory changes are intended to improve access to medication-assisted treatment and overall medical outcomes.
Let’s explore each of the key changes to 42 CFR Part 8 and dive into what healthcare professionals need to know.
Title and Terminology
SAMHSA’s update, renaming the rule to “Medications for the Treatment of Opioid Use Disorder,” reflects a commitment to evidence-based practices, embracing patient-centered terminology and approaches. Outdated, old-school medical terminology is out and easy-to-understand language is in.
Admissions
By eliminating barriers like the 1-year addiction history requirement or obtaining consent electronically and prioritizing pregnant individuals’ treatment, SAMHSA continues to foster inclusivity and streamline access to care, ensuring vulnerable populations receive the medical attention they need.
Treatment Standards
The integration of shared decision-making and harm reduction principles highlights SAMHSA’s dedication to meeting patients where they are in their recovery journey and helps pave a clearer path to positive change.
Take-Home Doses
The expansion of criteria for take-home doses of methadone acknowledges the success demonstrated during the COVID-19 pandemic, emphasizing patient independence while maintaining safeguards against diversion.
A survey revealed that the incidence of methadone diversion remains low among individuals receiving take-home doses under the COVID-19 Public Health Emergency flexibility measures. Additionally, an in-depth analysis of fatal overdose data spanning from January 2019 to August 2021 indicated that the implementation of this flexibility did not contribute to an increase in methadone-related deaths.
Telehealth
SAMHSA’s endorsement of telehealth for buprenorphine and methadone initiation aligns with evidence-based practices, enhancing access to care and supporting activities that contribute to recovery, such as employment. This update supports the notion that telehealth has effectively demonstrated its efficacy and safety in numerous healthcare settings, including the treatment of substance use disorders.
Interim Treatment
Expanding access to interim treatment options, coupled with extended time frames (from 120 to 180 days), ensures individuals receive timely care, prioritizing their journey toward comprehensive treatment.
Accreditation Body Oversight
Clarified responsibilities for accrediting bodies enhance monitoring and uphold quality standards across OTPs, ensuring adherence to regulatory guidelines.
OTP Compliance and Accreditation
Streamlined processes and extended corrective action timelines (180 days following receipt of the survey report) provide OTPs with the necessary flexibility to maintain compliance while ensuring continuity of operations.
Scope of Practice Expansion
Empowering nurse practitioners and physician assistants to order medications for OUD improves access to care, especially in underserved areas, while clarifying medication unit rules enhances service delivery.
Understanding SAMHSA’s Methadone Take-Home Supply Flexibilities Extension Guidance
Broadening the parameters for methadone take-home doses via telehealth spotlights a commitment to patient autonomy while enhancing access to care. SAMHSA’s Methadone Take-Home Flexibilities Extension Guidance represents a significant change in the landscape of OTPs across the United States. This exemption, depending on state agreement, offers more autonomy to OTPs in providing unsupervised take-home doses of methadone to patients with OUD.
This exemption will be in effect for one year after the end of the COVID-19 Public Health Emergency or until the U.S. Department of Health and Human Services completes updated regulations. It replaces earlier guidance and shows SAMHSA’s dedication to improving patient-centered care.
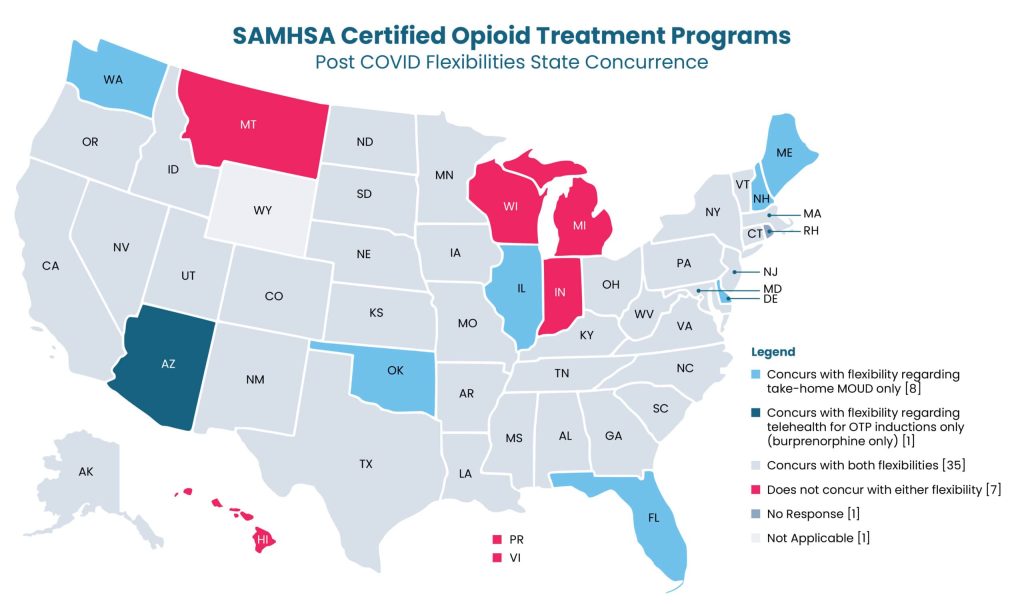
States differ in their acceptance of these options, with some supporting both take-home medication and telehealth for OTP introductions, while others are more hesitant or haven’t responded to the matter.
For those states that are on board, SAMHSA stresses the significance of healthcare providers exercising clinical judgment in deciding if patients qualify for unsupervised doses. Key criteria include the absence of substance use disorders, regular attendance, and no recent diversion activities. By recording these determinations in patients’ medical files, healthcare providers ensure accountability and compliance with regulations.
By allowing for increased flexibility in take-home medication via telehealth provisions, SAMHSA is taking a massive leap toward the evolving needs and preferences of patients. This decision also promotes a higher level of trust and furthers the relationship between patient and practitioner. As healthcare providers navigate these changes, the integration of multi-disciplinary clinical teams and informed discussions regarding patient safety remain of the utmost importance.
Landmark Changes to Regulations Governing OTPs
As the opioid crisis continues to wreak havoc across the nation, the Health and Human Services Department took decisive action to address the need for effective treatment options. On February 2, 2024, a landmark final rule was issued.
This final rule brings about significant changes to regulations governing (OTPs and the provision of Medications for Opioid Use Disorder (MOUD). These changes, stemming from the Consolidated Appropriations Act of 2023, not only make COVID–19–related flexibilities permanent but also remove the rigid requirements associated with the Drug Addiction and Treatment Act (DATA) Waiver. One of these changes is the integration of telehealth provisions, including the use of audio-visual and audio-only platforms for patient evaluations and medication initiation.
The effective date of the final rule, April 2, 2024, marks a turning point in the treatment landscape for OUD. Healthcare professionals will see a seismic shift in OTP accreditation, certification, and treatment standards. With the compliance date set for October 2, 2024, the future of OUD care looks brighter than ever before.

This groundbreaking rule builds upon decades of research and practice-based evidence, incorporating lessons learned from the COVID–19 pandemic. By offering telehealth flexibility for buprenorphine initiation and expanding access to methadone therapy, the Department aims to reach remote and underserved communities. The removal of DATA Waiver requirements signifies a more accessible and patient-centered approach to MOUD treatment.
As practitioners adapt to the new regulatory requirements, collaboration, compliance, and commitment to evidence-based care will be crucial to addressing the numerous challenges of OUD treatment. To do so, healthcare professionals should familiarize themselves with the full list of changes outlined and prepare to integrate telehealth into their OUD treatment protocols to enhance patient care.
Conclusion
The common thread that ties all these revisions together is providing accessible patient care. Additionally, it emphasizes the benefit of telehealth amid the evolving landscape of healthcare delivery for its ability to facilitate greater accessibility to treatment and align with the diverse needs and circumstances of patients seeking care for OUD.
By embracing these changes, healthcare professionals have the opportunity to optimize patient experiences and treatment outcomes. At Mend, we recognize the importance of seamless patient engagement and telehealth experiences. Our solutions automate workflows, facilitate digital patient intake, and reduce no-shows, empowering healthcare professionals to focus on delivering exceptional care.
In light of SAMHSA’s revisions to 42 CFR Part 8, healthcare professionals serve as the catalyst for creating meaningful OUD treatment change, reducing opioid dependence, and eradicating substance abuse. By embracing innovation, fostering inclusivity, and prioritizing patient-centered care, healthcare professionals pave the way for a future where access to quality treatment is within the realm of possibility for those affected by opioid addiction.