Aging and the New Home Health, Tech-Enabled – Learning from Nature
Health Populi
JULY 20, 2023
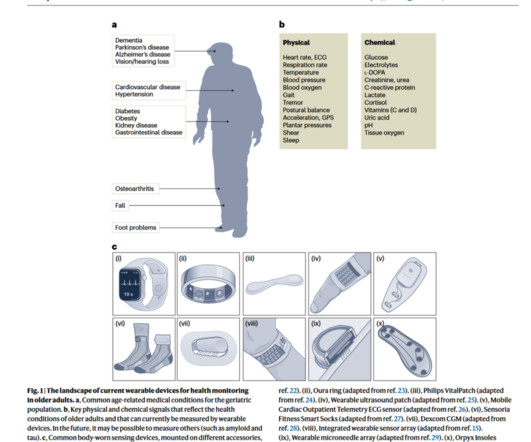
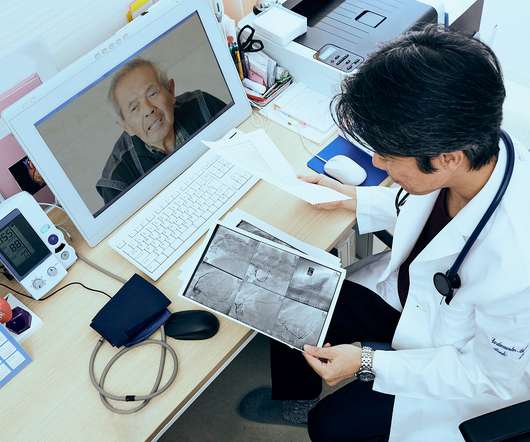
“Digital technologies offer tremendous potential for shifting from traditional medical routines to remote medicine,” with the role of wearables playing a growing role in the new home care for healthy aging. But what are the challenges of deploying this promising tech with older people keen to be independent at home?












































Let's personalize your content