My colleague, Christoph Brieden, recently presented on Perficient’s WHODrug Koda Interface at a webinar hosted by the Uppsala Monitoring Centre (UMC), which was attended by over 200 people. If you didn’t see the webinar and have a UMC login you can access a recording here.

Although still at the prototype stage, we are keen to gauge potential interest in our WHODrug Koda Interface. I am pleased to continue to spread the word at the ACDM20 Conference in Dublin on March 9th and 10th. I will be presenting on Tuesday, March 10th, on the integration of WHODrug Koda with medical coding systems, during the 11:35am to 12:25pm Parallel Session A.
The interface will enable a direct connection between medical coding systems and the UMC WHODrug Koda coding engine. The UMC used Vigibase to train Koda and our initial investigation of the Koda capabilities showed really promising results, as you would expect. We believe Koda could be of great benefit to the coding community by taking advantage of Artificial Intelligence to handle the ever-increasing volumes of data to code.
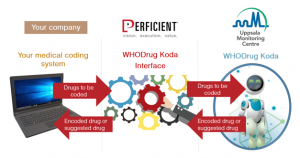
If you would like to discuss the possibilities of integrating Koda into your medical coding process, I’d be happy to discuss this further at the conference following my presentation or at the social gathering touring the Guinness factory!
Alternatively, please contact us so we can network:
Caz Halsey, Director, Life Sciences: Caz.Halsey@Perficient.com
Rudolf Coetzee, Business Development, Life Sciences: Rudolf.Coetzee@Perficient.com

