T1D Delay/Prevention in at-risk Children
Belgian company doses patients in phase Ib/IIa clinical trial with a unique approach to prevent or delay T1D onset

We spoke with Pieter Rottiers, Ph.D., CEO of ActoBio Therapeutics.
Genetic testing, combined with existing NIH TrialNet procedures, has shown very positive results in predicting T1D (see article). Intervention at the earliest possible time is expected to improve outcomes for those predisposed toward Type 1 diabetes. Early diagnosis and standard of care in T1D traditionally focuses on prolonging the honeymoon period wherein the pancreas continues to produce sufficient insulin.
ActoBio Therapeutics™ is pioneering a new approach with ActoBiotics® AG019, an orally delivered therapeutic agent designed to reverse the progressive autoimmunity that underlies T1D. AG019 shows potential to induce immune tolerance, thereby delaying or completely preventing the progression of the autoimmune destruction of pancreatic beta cells, preserving full insulin capabilities. A clinical trial is ongoing and accepting new patients (https://clinicaltrials.gov/ct2/show/NCT03751007).
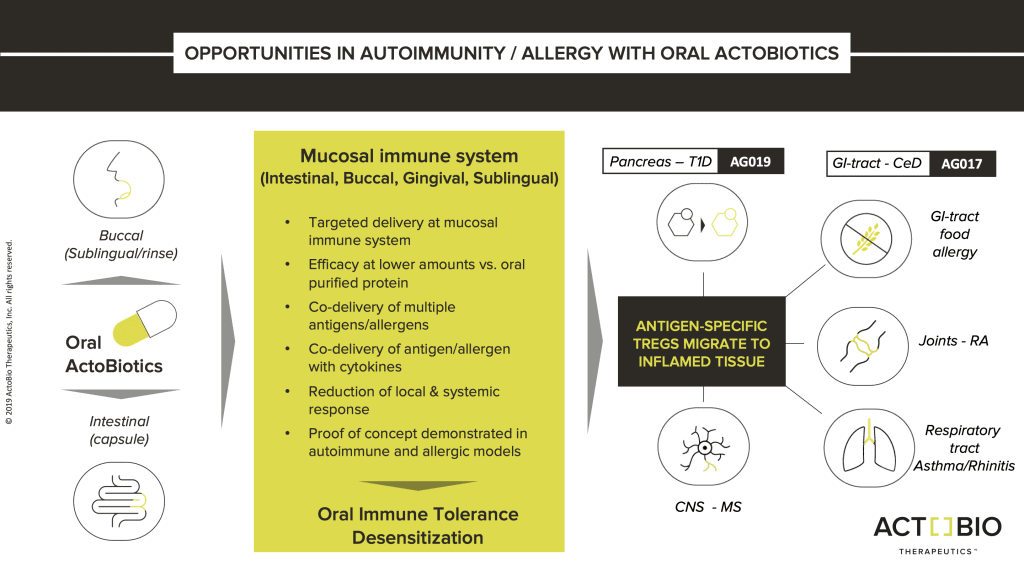 Proprietary platform
Proprietary platform
ActoBiotics® is a new class of biopharmaceuticals based on ActoBio’s proprietary platform of engineered food-grade microbes (Lactococcus lactis) that can express cytokines, enzymes, hormones, and monoclonal antibodies as therapeutics within the body.
Lactococcus lactis is a Gram-positive bacterium used extensively in the production of many foods like buttermilk and cheese and therefore has a long history of safe use. In 2006 it became the first live genetically modified bacterium to be used in clinical trials to deliver a human therapeutic protein for the treatment of human disease, by Pieter Rottiers Ph.D., CEO of ActoBio, while he was working at Gent University (https://www.ncbi.nlm.nih.gov/pubmed/16716759).
The ActoBiotics® platform offers the ability to deliver biological effectors selectively to mucosal tissue sites, such as the oral and gastrointestinal tract. With intervention focused at the mucosal immune system and/or site of pathology, this ground-breaking class of orally available biopharmaceuticals has the potential to facilitate targeted therapies against oral, gastrointestinal, metabolic, allergic and autoimmune diseases.
AG019 presents proinsulin to the body in much the same way as happens with food antigens. Following ingestion, an oral capsule releases specifically designed L. lactis that now produce the autoantigen proinsulin at the intestinal mucosal immune system. This is expected to induce tolerance to proinsulin and potentially provides a disease-modifying treatment.

“We are focused on therapy delivery via the gastrointestinal tract which is the largest immune organ in the body.” — Pieter Rottiers, Ph.D., Chief Executive Officer of ActoBio Therapeutics
Treatment specifics
AG019 is formulated as a capsule consisting of genetically engineered Lactococcus lactis that is specifically modified to secrete human proinsulin and the tolerance-enhancing cytokine human interleukin-10 to the mucosal lining of the gastrointestinal tissues.
The microbe-based platform offers several advantages over injectable biologics, including a unique delivery method of a therapeutic agent capable of inducing oral immune tolerance to reverse T1D.
Treatment Regimen
Each capsule dissolves in the digestive system and the dry contents rehydrate. The active ingredients are then produced at the intestinal mucosal immune system and have an effective useful lifetime of 48-72 hours during transit until they are discharged from the intestine. Importantly, the engineered Lactococcus lactis does not colonize the GI tissue so the dosage is fully controllable.
The treatment is designed so a person would take capsules for two months and that would conclude their therapy. At that point, a cure would be achieved if C-peptides are stable, indicating that the body is producing insulin normally in the pancreas.
![]() It is possible that a booster might be required at some future time but it is unknown whether this might be required in one year or ten years.
It is possible that a booster might be required at some future time but it is unknown whether this might be required in one year or ten years.
“ActoBio’s AG019 shows real potential for stemming the progression of type 1 diabetes and, in combination with new methods of identifying at-risk populations, may provide an exciting opportunity to prevent clinical onset.” — Kevan Herold, MD, Professor of Immunobiology and of Medicine (Endocrinology) at Yale University
Clinical trial
A phase Ib/IIa clinical trial started in late 2018 and will continue across a number of clinical sites specializing in the treatment of T1D in both the US and Europe. Results are expected at the beginning of 2020 and will provide data regarding the safety and efficacy of AG019 in humans.
“This trial is a critical next step in what we believe will be a very exciting journey in the development and assessment of a novel treatment option for early-onset T1D.” — Pieter Rottiers, Ph.D., Chief Executive Officer of ActoBio Therapeutics
Company
ActoBio Therapeutics, Inc. — http://www.actobio.com/ — is a wholly owned subsidiary of Intrexon Corporation (NASDAQ: XON) — https://www.dna.com/
The predecessor company to ActoBio was established in 2006. They applied for licences to develop these genetically modified live bacteria from the FDA and the EMA (FDA’s equivalent in the European Union), who developed a new process to approve this kind of delivery system. ActoBio was acquired by Intrexon in 2015 and gained IND approval for AG019 for type 1 diabetes in 2018.
ActoBio has active Investigational New Drug programs (INDs) with the FDA and Clinical Trial Agreements (CTAs) with the EMA. INDs and CTAs are the means by which a pharmaceutical company obtains permission to start human clinical trials. These currently include ongoing trials in AG019 for T1D and AG013 (in collaboration with Oragenics, Inc.) for oral mucositis.